Improve Protocol Design and Avoid Amendments by Expanding Your Stakeholder Network
Engage an extended operational perspective for a resource of insights into simplifying protocol design.
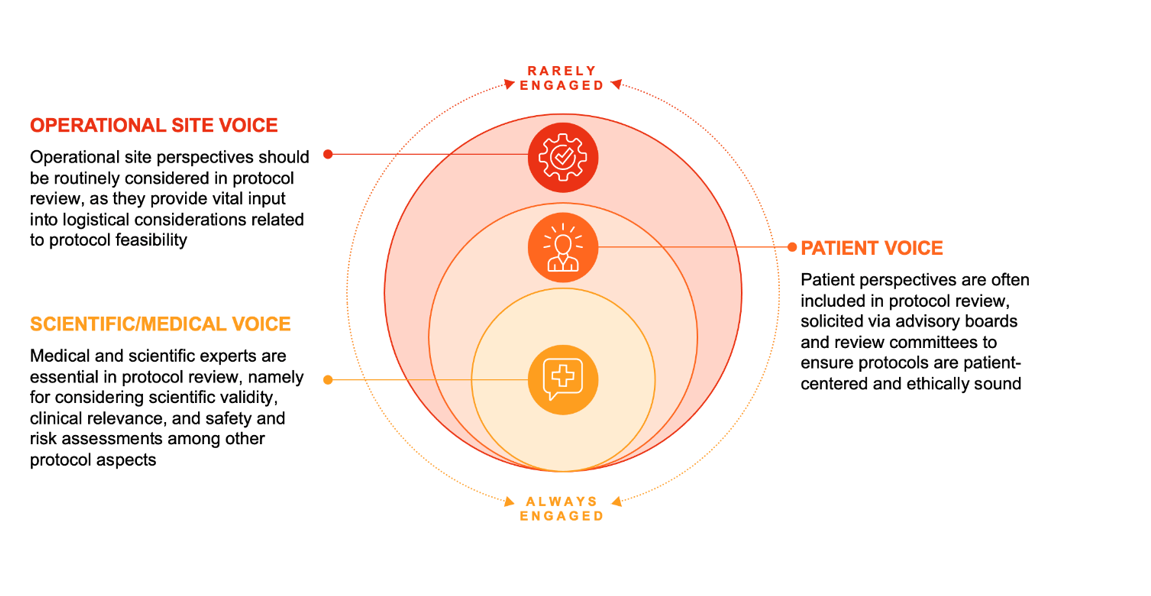
Complex clinical trial protocols are an industry-wide challenge seen across all therapeutic areas, sponsors, and trials. While the frequency of protocol amendments varies, evidence indicates a strong link between protocol complexity and the number of required amendments1, which results in unplanned expenses and delayed study timelines. Amendments directly impact sites and patients, requiring additional training and time to adapt to changes in the protocol design. Yet, amendments can be avoided, with an estimated half deemed preventable1. Biopharmaceutical companies strive to reduce protocol complexity, and thus amendments, through vigorous review processes, including internal medicinal and scientific experts, and patient advisory boards. Rarely do they go as far as engaging site staff. Yet, expanding the protocol review board provides an operational perspective and represents an untapped resource of insights into simplifying protocol design.
To expand this operational lens, sponsors can:
- Look to CRAs, research coordinators, and nurses through establishment of external partnerships (e.g., advisory boards, review committees).
- Engage site staff on trial elements that medical and scientific advisors may not have the knowledge to properly evaluate, e.g., addressing cultural and regional nuances of a clinical trial, and evaluate the operational burden on site staff.
- Provide practical perspectives to study feasibility by assessing recruitment strategies, providing input on potential challenges in identifying and enrolling eligible participants and evaluating inclusion and exclusion criteria.
- Evaluate required training and support needs for the study to assess the sufficiency of training requirements and required technical support for trial-related systems and procedures.
Learn more insights into simplifying protocol design.
Timing is Everything: When to Involve Key Stakeholders in the Protocol Process
Involving external stakeholder perspectives is only effective when conducted early in the protocol review process. Most sponsors tend to share near finalized protocols with patients and KOLs/PIs, limiting meaningful feedback. Early inclusion of the patient perspective in protocol reviews helps sponsors prevent issues like enrollment difficulties and high dropout rates, leading to amendments. This is important in predicting overall trial success, as challenges to patient recruitment and retention significantly delay study timelines and cause unexpected operational costs to increase over a clinical trial. Integrating patient feedback early in the process ensures that patient perspectives are effectively considered throughout trial design, implementation, and early dissemination into planning activities. Sponsors can look to engage with study committees, research networks, and advisory groups to gain these intended benefits.
Early inclusion of PIs and KOLs in the protocol review process is also crucial early in the process, as their provided expertise ensures that the protocol is scientifically sound, feasible and aligned with clinical practice. By involving these stakeholders earlier, sponsors can avoid feasibility and recruitment related amendments (e.g., assessing the site selection criteria to ensure they have necessary expertise and patient population).
Best practices for incorporating external stakeholders into the protocol review process, as provided through successful literature examples3, are as follows:
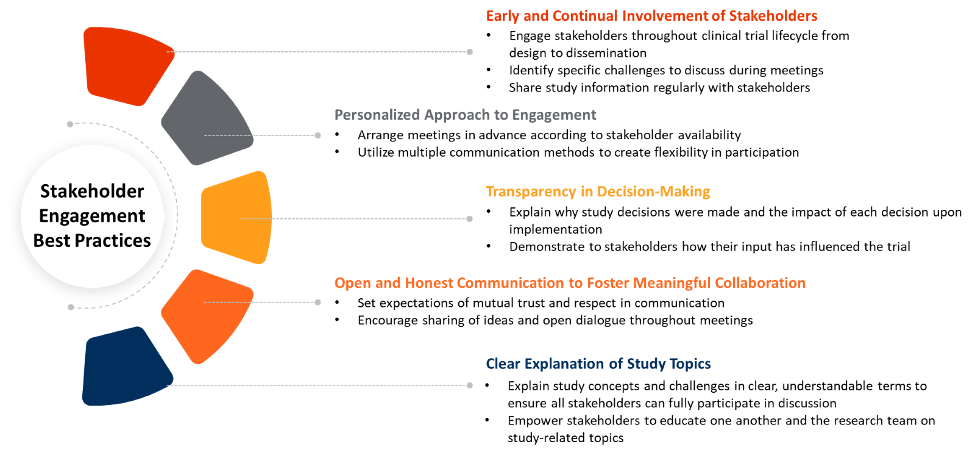
Given the anticipated rise in protocol complexity and the increased competition for sites and patients across the clinical trial landscape, sponsors should solicit early feedback from all stakeholder groups in protocol design to reduce operational complexity and enhance the value proposition of their clinical trial. Protocols that limit site and patient burden and are validated by key stakeholders will be particularly attractive for recruitment purposes. Sponsors will be able to limit extraneous operational costs and prevent extensive delays in trial timelines through reduced protocol amendments, effectively addressing both internal and external challenges that are present today.
Are you interested in simplifying your protocol design to reduce operational complexity and enhance your clinical trial value proposition? Learn more about how Syneos Health can help you optimize clinical trials from Phase I and beyond.
Contributors
Hailey Durham | Management Consultant, R&D Advisory Consulting
Jessie Tucker | Engagement Manager, R&D Advisory Consulting
Charlie Bergqvist | Director, R&D Advisory Consulting
Contact Us
References
- Getz, K.A., Stergiopoulos, S., Short, M. et al. The Impact of Protocol Amendments on Clinical Trial Performance and Cost. Ther Innov Regul Sci 50, 436–441 (2016). https://doi.org/10.1177/216847901663227
- Getz KA, Wenger J, Campo RA, Seguine ES, Kaitin KI. Assessing the impact of protocol design changes on clinical trial performance. Am J Ther. 2008 Sep-Oct;15(5):450-7. doi: 10.1097/MJT.0b013e31816b9027. PMID: 18806521.
- Barger S, Sullivan SD, Bell-Brown A, et al. Effective stakeholder engagement: design and implementation of a clinical trial (SWOG S1415CD) to improve cancer care. BMC Medical Research Methodology. 2019 Jun;19(1):119. DOI: 10.1186/s12874-019-0764-2. PMID: 31185918; PMCID: PMC6560751.



