Improving Racial and Ethnic Diversity in Alzheimer’s Disease Clinical Trials
Despite the complexities, clinical research partners are discovering innovative strategies for enhancing inclusivity in Alzheimer’s disease.
Alzheimer’s disease (AD) is a progressive neurodegenerative condition that is increasing in prevalence in patients over 65 and cost at unsustainable levels. There is evidence from available studies that show, in the US, Blacks and Hispanics are at higher risk; however, these populations represent a small proportion of patients enrolled in clinical trials. The underrepresentation of these groups in clinical trials highlights the urgent need for inclusive research practices as both ethical and scientific imperatives.
Global Regulatory Agencies Increasing Focus on Racial and Ethnic Diversity
Figure 1: US map depicting the number of AD patients compared to the relative diversity of insurance claims.
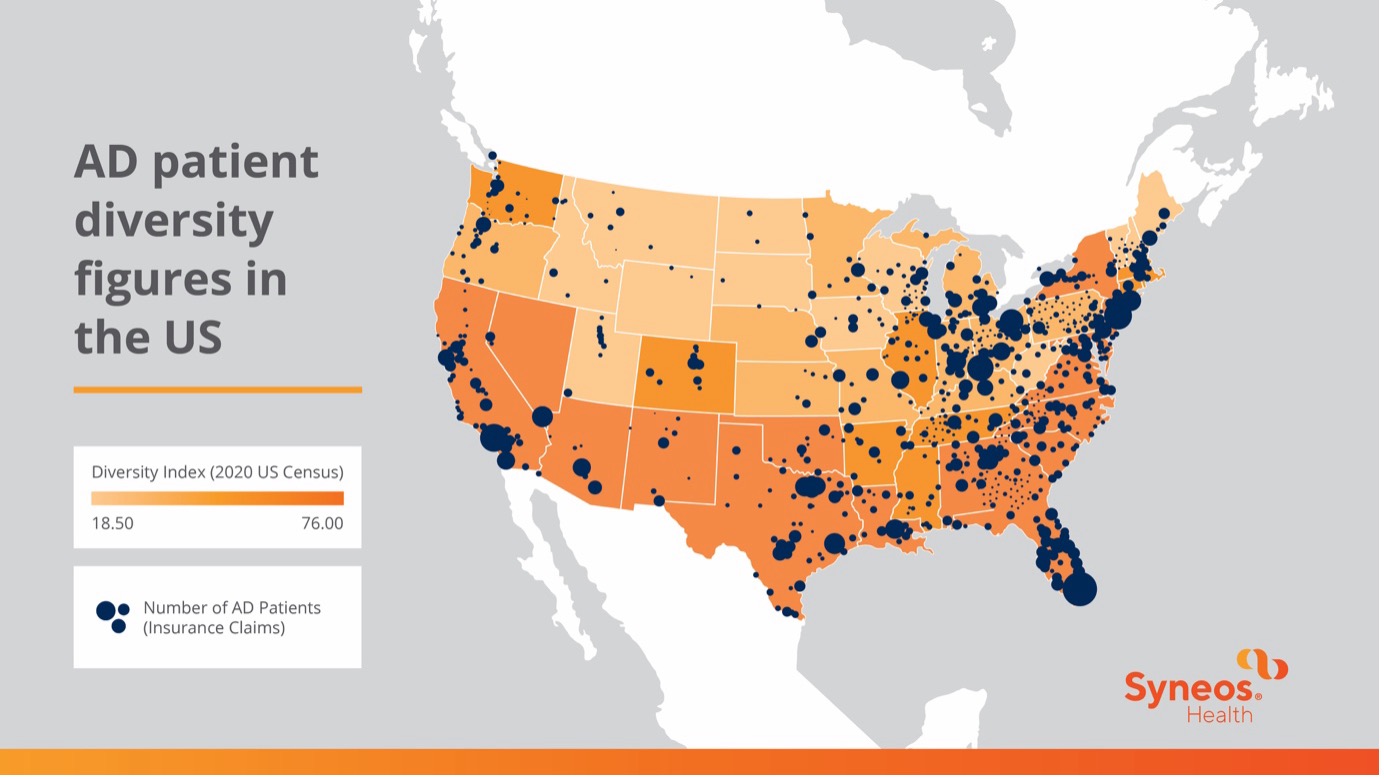
Efforts by global regulatory agencies to address racial and ethnic diversity in clinical research have gained momentum. In the US, the Clinical Treatment Act ensures Medicare coverage for clinical trial participants, expanding access to diverse populations. The FDA and other regulatory bodies have issued guidance and legislation advocating for increased diversity in clinical trial populations, requiring sponsors to develop diversity action plans and promote data modernization and transparency. With the FDA leading the charge in securing diversity goals, these initiatives signify a pivotal step towards equitable representation in clinical research on a global scale.
Challenges in achieving diversity in AD clinical trials
Despite these initiatives, there are multiple hurdles in the way of achieving racial and ethnic diversity in AD clinical trials:
- Challenging protocol designs: Achieving diversity in study designs remains trying, centered around meticulous protocols. This can lead to exclusionary eligibility criteria that can hinder accessibility for any patient participants, study partners and caregivers unable to make frequent site visits, exacerbating disparities in trial participation that inadvertently excludes specific comorbidities.
- Lack of inclusivity in awareness efforts: Recruitment and retention strategies in frequently overlook the importance of inclusive communications and advertising, failing to consider health literacy and plain language, "looks like me" campaigns and accessible platforms for information dissemination. Furthermore, while implicit biases further impede recruitment efforts, study designs often lack patient-centric components of decentralized trials.
- Insufficient community outreach: Efforts in community education often struggle to effectively raise awareness about ongoing studies, neglecting to consider the diverse social, cultural, and economic backgrounds of potential participants. Historical events have also fostered deep-seated mistrust within certain communities, complicating efforts to engage these populations in clinical research.
- Lack of specific country and site selection: Additionally, when considering countries for clinical trials, sponsors often fail to adequately represent the local population and grapple with limited demographic data.
- Lack of site staff diversity: Diversity among site staff remains an ongoing issue, with clinical investigators and their teams often lacking representation from diverse backgrounds. Furthermore, site staff may lack awareness of regulatory requirements for inclusivity and cultural competency, hindering effective communication with participants from varied backgrounds.
To improve diverse enrollment overall, clinical research partners need to have a specific strategy aimed at improving diversity in AD trials, including:
- Utilizing the latest disease incidence and prevalence data by race and ethnicity to inform clinical trial design and ensure representation in all activities in site selection.
- Partnering with stakeholders across the clinical trial ecosystem (including patient groups, community members, research sites, clinical research organizations (CROs), academia, nonprofit and advocacy organizations and federal and state agencies, to gather insights and establish patient-centric designs that support patients, caregivers and study partners.
- Building relationships with target communities through community leaders to provide outreach, training and education, fostering trust and engagement.
- Involving diverse populations in developing patient-friendly resources simplifying the process of identifying and enrolling in relevant clinical trials.
- Leveraging innovative strategies to enroll and retain diverse participants such as decentralized clinical trials (DCTs), expanded office hours, improved reimbursement processes and enhanced accessibility measures, ensuring equitable access for all participants.
- Creating diverse site teams and ensuring clinical trial diversity and cultural competency training for staff to understand better and support diverse patient communities.
A well-designed approach to AD trial diversity involves creating tailored diversity strategies that account for patient geographic distribution, patient experiences and insights, and involvement in protocol design. The right approach will also provide community engagement and diversity recruitment support to sites, aiming to alleviate the burden of trial participation and execute enrollment and retention practices that promote inclusivity for all patient populations who may benefit from intervention.
Ready to make a difference in Alzheimer's disease care? Explore our therapeutically aligned AD solutions.
Contributors
Claudine Brisard | SVP, Medical and Scientific Management
Batisha Anson | Global Head, Patient Diversity and Health Equity



