Medical Device and Diagnostics
Specialized support to accelerate clinical development and commercialization
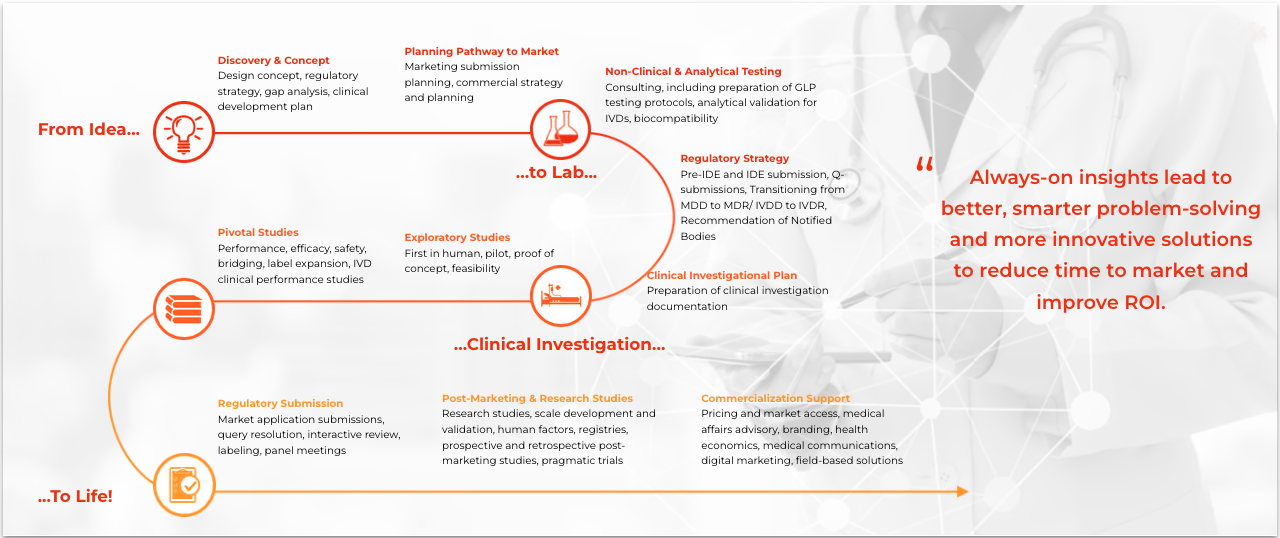
Medical device and diagnostics research is a broad and increasingly complex area requiring specialized expertise. Our focused group of medical device and diagnostics (MD&D) experts offer a full breadth of MD&D-specific capabilities that span the entire nonclinical and clinical development, regulatory, medical affairs and commercial continuum to strategically accelerate the product development lifecycle.
Medtech clinical trial solutions tailored to your requirements
As a medical device CRO, we thrive on collaboration. Together, we will review your plan, take the time to understand your goals, and aim to develop the most cost- and time-efficient development strategy to meet clinical, regulatory, and market adoption requirements.
Whatever your needs, we will work with you to adopt new processes and technologies, leverage our diverse therapeutic expertise, and access commercial insights. The goal? Facilitating shorter and more efficient clinical trials designed to improve the likelihood of regulatory and commercial success.
Trusted Process
This is our proprietary, metrics-driven project management methodology initially developed to manage all aspects of your clinical investigations. This unique, four-step approach delivers faster results while maintaining data integrity and reducing operational risk and variability. It provides operational framework for every aspect of the work we undertake for you and with you. Read more.
Synthesized Data-Generating Customized Insights
Your business is evolving faster than ever before, and so is technology. We believe the right strategy is to leverage data and technology from data lakes from diverse sources. We call this Dynamic Assembly®. Augmented by internal expertise of our teams, we can support site identification, country validation and enrolment projection decisions.
Read more.
Relationship with the Sites
We are voted as a top CRO to work with. Our sites collaborate with us, and we will use these relationships while delivering your clinical investigation. Read more.
Integrated Quality Risk Management (IQRM)
We apply this approach to your project, which provides identification of and planning for successful risk mitigation strategies.
Risk-Based Monitoring
We customize risk-based monitoring strategies for each study to assure data integrity. Our processes comply with ISO 14155:2020 for medical devices and ISO 20916:19 for in-vitro diagnostics.
Syneos Health Therapeutic Experience
We are built on our firm belief in therapeutic depth. We assemble teams in which key members, from CRAs to senior management, are experts in devices and diagnostics. This team approach delivers much stronger insights into the therapeutic and operational environment.

Strategy driven by a clear view of the regulatory landscape
Our customized clinical development solutions are paired hand-in-hand with bespoke regulatory solutions.
Our boutique MD&D CRO team has global muscle: our Global Regulatory Affairs Solutions (GRAS) experts provide full-spectrum, end-to-end solutions to biopharmaceutical, medical device, in vitro diagnostic (IVD) and combination product companies worldwide.
Our GRAS experts will lead you through important regulatory activities related to discovery, surveying the regulatory landscape, strategic planning, clinical investigation submissions, regulatory authorization submissions, maintenance, post-marketing requirements and compliance.
Our GRAS experts are located in regional centers of excellence in North America, Europe and Asia-Pacific, offering timely regulatory advice for every stage of your product’s lifecycle.
Our regulatory capabilities include:
Discovery & Concept
- Design concept to meet unmet need and proof of concept
- Identify regulatory pathway and requirements
- Gap Analysis
- Clinical Development Plan
- Pre-Submission
- Health Authority and Notified Body Meetings
Pathway to Approval
- Market approval submission planning for 510(k)/DeNovo
- CE Marking technical files
- Clinical evaluation reports
- General Safety and Performance Checklists
- Health Authority and Notified Body Meetings
Regulatory Review
- Market application submissions to regulatory agencies
- Submissions to Notified Bodies
- Query Resolution
- Interactive review
- Labeling
- Ad Com Meetings
Regulatory Maintenance
- Supplements
- Amendments
- Annual reports
